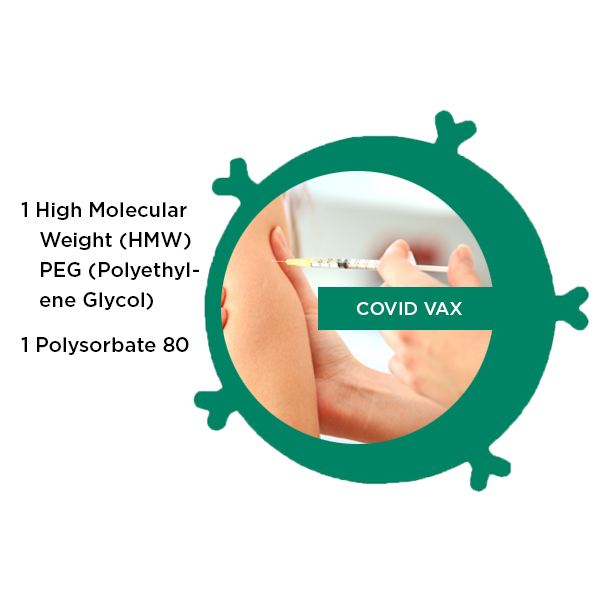
COVID Vax Panel
$510.00
One third of the US population has received the COVID vaccine. If you are in the two thirds that have not received it, you may be considering it, weighing the pros and cons. While the risk of severe allergic reaction is very low, it is likely one of the factors you are considering.
The CDC and the vaccine manufacturers caution that known allergy to “any component” is a contraindication to the vaccines. For the COVID-19 vaccines, the components implicated in hypersensitivity are high molecular weight polyethylene glycol (PEG 2000) and Polysorbate 80.
All guidance clearly states that individuals with known allergy to PEG should not receive the Pfizer or Moderna vaccines. Those with allergies to Polysorbate 80 should not receive the Johnson & Johnson vaccine. Because the two ingredients are closely related, if you have a known allergy to either, you should consult with your healthcare practitioner for guidance before getting any of the COVID-19 vaccines.
If an immediate allergy to either ingredient is suspected, a skin scratch tests can be performed. These conventional tests identify immediate allergy (type 1 immune hypersensitivity).
For information about delayed reactions to these two vaccine components, LRA tests are now available.
COVID Vax LRA Panel test items:
High Molecular Weight (HMW) polyethylene glycol PEG. Moderna and Pfizer COVID 19 vaccines include HMW PEG.
Polysorbate 80 – Used in the Johnson and Johnson COVID 19 vaccine.
The LRA uses an advanced ex-vivo cell culture method to simultaneously detect all three types of delayedhypersensitivities (Types II, III, and IV). Unlike immediate allergies, these delayed hypersensitivities, also called delayed allergies, can occur any time between 3 hours and 3 weeks after exposure. This delayed reaction makes it difficult to identify the triggering substances without a highly accurate test like the LRA. Learn more.
One third of the US population has received the COVID vaccine. If you are in the two thirds that have not received it, you may be considering it, weighing the pros and cons. While the risk of severe allergic reaction is very low, it is likely one of the factors you are considering.
The CDC and the vaccine manufacturers caution that known allergy to “any component” is a contraindication to the vaccines. For the COVID-19 vaccines, the components implicated in hypersensitivity are high molecular weight polyethylene glycol (PEG 2000) and Polysorbate 80.
All guidance clearly states that individuals with known allergy to PEG should not receive the Pfizer or Moderna vaccines. Those with allergies to Polysorbate 80 should not receive the Johnson & Johnson vaccine. Because the two ingredients are closely related, if you have a known allergy to either, you should consult with your healthcare practitioner for guidance before getting any of the COVID-19 vaccines.
If an immediate allergy to either ingredient is suspected, a skin scratch tests can be performed. These conventional tests identify immediate allergy (type 1 immune hypersensitivity).
For information about delayed reactions to these two vaccine components, LRA tests are now available.
COVID Vax LRA Panel test items:
High Molecular Weight (HMW) polyethylene glycol PEG. Moderna and Pfizer COVID 19 vaccines include HMW PEG.
Polysorbate 80 – Used in the Johnson and Johnson COVID 19 vaccine.
The LRA uses an advanced ex-vivo cell culture method to simultaneously detect all three types of delayedhypersensitivities (Types II, III, and IV). Unlike immediate allergies, these delayed hypersensitivities, also called delayed allergies, can occur any time between 3 hours and 3 weeks after exposure. This delayed reaction makes it difficult to identify the triggering substances without a highly accurate test like the LRA. Learn more.